Explainer: All crude oil is not alike
Oil companies prefer the lighter, ‘conventional’ oils, from which it is easier to make gasoline and other desirable products

Much of the crude oil extracted from the Earth today is brought up from underground wells with pumps such as this one.
tifonimages/iStockphoto
By Janet Raloff
Geologists describe petroleum as it comes out of the ground as crude oil. It can vary dramatically from place to place. Some of it is relatively thin. It may pour about as easily as the oil used to lubricate auto engines. These oils are known as conventional crudes. But there is also a broad range of crude oils considered unconventional. They can be thick — even tarlike. In the most extreme cases, they will pour about as easily as peanut butter (which means that at room temperature they really won’t pour at all).
All crude oil is made up of a complex mix of molecules known as hydrocarbons. There are many dozens of these molecules. They get their name from the fact that they are combinations of hydrogen and carbon atoms that nature has chemically bound together.
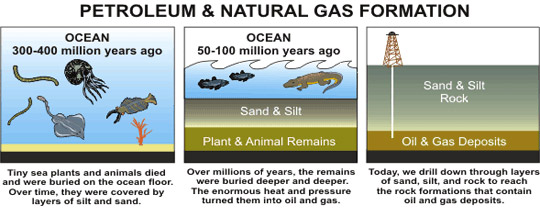
Petroleum hydrocarbons developed out of the decaying remains of plants and animals that lived throughout the world’s oceans before the time of dinosaurs. Over millions of years, they became deeply buried under layers of sand and sediment. Compressed by the weight (pressure) of those upper layers, the decaying remains heated up. Later, they underwent chemical reactions. Heavy crudes developed where microbes were able to feast on and remove the smaller hydrocarbons. That left behind only big (and heavy) molecules.
Chemical companies prefer the thinner crude oils. They can more easily convert these — through processes known as refining — into products that consumers prize. These include gasoline, diesel fuel, jet fuel, kerosene and petrochemicals. (That last group includes organic solvents and plastics). Conventional oils tend to come from the ground with fewer natural impurities (such as sulfur and metals). Those must be removed prior to refining.
“Conventional light oil can typically be produced at a high rate and a low cost,” note Richard F. Meyer and Emil D. Attanasi. They work for the U.S. Geological Survey. It’s in Reston, Va. That’s why oil companies prefer to work with light crudes. But a heavy demand for products made from crude oil over the past 80 years has led oil companies to pump enormous amounts of crude oil from the ground. Many huge natural deposits of this oil have now been virtually emptied.
This has led oil companies to take a new interest in the heavier, hard-to-work-with types.
Among these are the so-called heavy crudes and bitumen (or tar sands). Venezuela’s Orinoco Basin in South America holds an estimated 90 percent of the world’s supply of recoverable heavy crude, Meyer and Attanasi report. Canada’s western province of Alberta is home to more than 80 percent of the world’s known (extractable) supplies of bitumen. This is a super-heavy crude about as thick as tar. Together, world supplies of these heavy and super-heavy crudes about equal the remaining volumes of usable conventional oils, the USGS researchers report.
So plenty of unconventional oil remains in the ground. However, the costs of mining and refining them into the products that people want is far higher than for light crudes. It also requires lots more energy. Maybe even more important, refining heavy crudes produces lots of pollution.
Power Words
bitumen (also known as tar sands or oil sands) A type of extra-heavy crude oil that is tarlike at room temperature.
conventional oil A crude oil that leaves the ground as an easy-to-pour natural mix of hydrocarbons.
diesel fuel A fuel obtained during petroleum refining or from blends of refining distillates mixed with residual oil used in motor vehicles. Diesel fuels are heavier than gasoline and have a higher boiling point.
gasoline A complex mixture of relatively volatile hydrocarbons with or without small quantities of additives. They are blended to form a fuel for use in spark-ignition engines.
hydrocarbons A group of many compounds made by linking together different numbers of hydrogen and carbon atoms. Examples include methane, propane and ethylene.
kerosene A thick oil obtained from petroleum and used both as a fuel and solvent.
organic A term that refers to a range of carbon-based molecules (other than carbon dioxide and carbon monoxide).
petroleum A term that usually refers to crude oil or products obtained by refining, or processing, crude oil.
production (of crude oil and gas) Moving oil and natural gas to the surface from underground reservoirs and then treating these to obtain liquid hydrocarbons for use as fuels or the ingredients to make commercial chemicals.
refining An energy-intensive industrial process that transforms crude oil into fuels such as gasoline and heating oil or into chemicals used to make plastics, synthetic rubber, solvents and a range of other products.
unconventional oil A crude oil that leaves the ground as an especially thick (viscous) natural mix of hydrocarbons.







