Physics
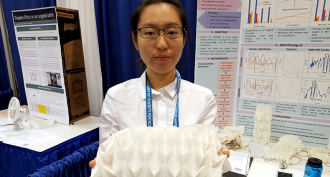
Musicians could benefit from teens’ research that pictures sound
While in middle school, Hannah Shu and Isabelle Katz developed ways to picture musical tones. Their research could help everyone from instrument shoppers to vocal coaches.
By Sid Perkins